- Chemical equation
- Question And Answers
- Chemical equation
- Law of conservation of mass
- Laws of constant proportion
- Dalton’s atomic theory
- Atoms and molecules
- Symbols of elements
- Atomicity
- Valency
- What is an Ion ?
- Atomic mass
- Molecules of compounds
- Molecular mass , Formula unit mass
- Mole
- Molar mass
- Types of Chemical Reactions
- Decompostion Reaction
- Displacement reaction
- Double displacement reaction
- Oxidation and Reduction
- Effects of oxidation reactions in daily life
- 7.Atoms, Molecules And Chemical Reactions
- Atoms, Molecules, and chemical reactions
- Classification of Elements
- Dobereiner’s law of Triads
- Limitations
- Mendeleeff’s Periodic Table
- Salient features and achievements of the Mendeleeff’s periodic table
- Limitations of Mendeleeff’s periodic table
- Modern Periodic Table
- Groups,
- Periods
- Metals and Non metals
- Atomic radius
- Periodic properties of the elements in the modern table
- Ionization Energy
- Electronegativity
- Metallic and Non-Metallic Properties
- Short answer Questions
- Question And Answers
- Synopsis
- Classification of elements - The Perodic Table
- Nutrition
- Mechanism of Photosynthesis
- Light independent reaction (Biosynthetic phase)
- Heterotrophic nutrition and nutrition in Human Beings
- Health aspects of the elementary canal
- Coordination in life processes
- Peristaltic Movement in Esophagus
- Taste connected with tongue and palate
- Villi
- Respiration -The energy Producing system
- Epiglottis and passage of air
- Gaseous Exchange
- Respiration versus combustion
- Transportation-The circulatory system
- The blood vessels and The cardiac cycle
- Blood pressure and Materials transport in the plants
- The wastage disposing system
- Mechanisms of urine formation
- Other Pathways Of Excretion
- Excretion release in substance of plants
- Short Answer Questions
- Long answer Questions
- Synopsis
- Reproduction - The generating system
- Reproduction in a placental mammal - Man
- Cell cycle
- Reproduction In Organisms
- Parthenogenesis
- Vegetative Propagation
- Male Reproductive System
- Female reproductive system
- STRUCTURE OF A SPERM
- Menstrual Cycle
- Extra Embryonic Membrane
- Sexual reproduction in flowering plants
- Synopsis
- Long Answer Questions
- Short Answer Questions
- Quizz
- Important Quiz Questions
- Introduction
- Lewis Electron Dot Symbols
- Ionic Compounds: Electrons Transferred
- Formation of Ionic Compounds
- The arrangement of ions in ionic compounds
- Covalent bond
- VSEPR theory
- Valence bond theory
-
Formation of O_2, N_2, CH_4Molecules
- Hybridisation
- Properties of ionic and covalent compounds
-
Formation of BF_3,NH_3, water molecule
- Synopsis
- Question And Answers
- Chemical Bonding
Chemical properties of Acids and Bases
Chemical properties of Acids and Bases
Response of various laboratory substances with indicators.
Collect the following samples from the science laboratory; hydrochloric acid (HCl), sulphuric acid (H_{2}SO_{4}
(CH_{3}COOH
Take four watch glasses and put one drop of the first solution in each one of them and test the solution as follows.
i)dip the blue litmus paper in the first watch glass.
ii)dip the red litmus paper in the second watch glass.
iii)add a drop of methyle organge to the third watch glass, and
iv)add a drop of phenolphthalein to the fourth watch glass.
Observe the respective colour changes and note down in Table. Do the same with all the above dilute solutions.
 There are some substances whose odour changes in acidic or basic media. These are called olfactory indicators.
There are some substances whose odour changes in acidic or basic media. These are called olfactory indicators.
Let us work with some of such indicators.
Reaction of Acids and bases with Metals
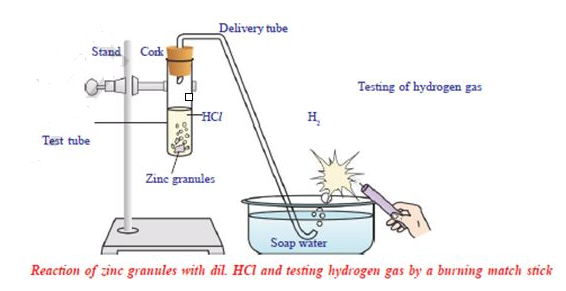 Materials required: test tube, delivery tube, glass trough, candle, soap water, dil. HCl, and zinc granules, cork.
Materials required: test tube, delivery tube, glass trough, candle, soap water, dil. HCl, and zinc granules, cork.
Procedure: Set the apparatus as shown in fig.
- Take about 10ml of dilute HCl in a test tube and add a few zinc granules to it.- What do you observe on the surface of the zinc granules?
- Pass the gas being evolved through the soap water.
- Why are bubbles formed in the soap solution?
- Bring a burning candle near the gas-filled bubble.
- What do you observe?
You will notice the gas evolved burns with a pop sound indicating H2.
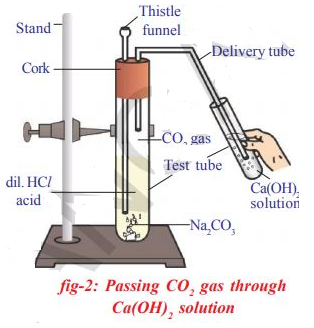
The chemical reaction of the above activity is:
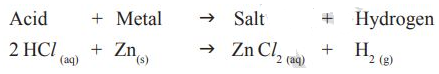
Reaction of Acids with carbonates and metal hydrogen carbonates
*.Take two test tubes; label them as A and Take about 0.5 gm of sodium carbonate (Na2CO3) in test tube A and about 0.5 gm of sodium hydrogen carbonate (NaHCO3) in test tube B.
*Add about 2 ml of dilute HCl to both
*What do you observe?
*Pass the gas produced in each case through lime water (calcium hydroxide solution) as shown in Fig 2 and record your observations.
The reactions occurring in the above activities are as follows:
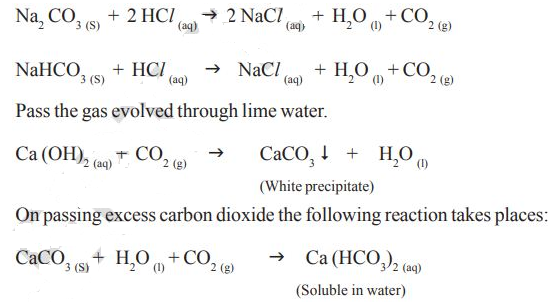 Thus from above activities you can conclude that the reaction of metal carbonates and hydrogen carbonates with acids give a corresponding salt, carbon dioxide and water. We can write generalized form of these chemical reactions as shown below:
Thus from above activities you can conclude that the reaction of metal carbonates and hydrogen carbonates with acids give a corresponding salt, carbon dioxide and water. We can write generalized form of these chemical reactions as shown below:
metal carbonate + acid J salt + carbon dioxide + water
metal hydrogen carbonate + acid J salt + carbon dioxide + water
Neutralization reaction
Acid – base (Neutralization) reaction Take about 2 ml of dilute NaOH solution in a test tube and add one drop of phenolphthalein indicator. Observe the colour of the solution.
Add dilute HCl solution to the above solution drop by drop. Is there any change of colour of the solution?
Why did the colour of the solution change after adding the HCl
solution?
-Now add one or two drops of NaOH to the above mixture.
•Does the Pink colour reappear?
Do you guess the reason for reappearance of pink colour? In the above activity you observe that the pink colour disappears on adding HCl because NaOH is completely reacted with HCl. The effect of base is nullified by an acid. Pink colour reappears on adding a drop of NaOH because the solution becomes basic once again. The reaction occuring between acid and base in the above activity can be written as:
NaOH+HCl \rightarrow NaCl+H_{2}O
The reaction of an acid with a base to give a salt and water is known as a neutralization reaction. In general, a neutralization reaction can be written as:
Base + Acid\rightarrow
Reaction of Acids with metal oxides
*Take a small amount of copper oxide (CuO) in a beaker and slowly add dilute hydrochloric acid while stirring. Observe the changes in the solution.
*Note the colour of the solution
What do you observe in the above reaction? You will notice that the copper oxide present in the beaker dissolves in dilute HCl and the colour of the solution becomes blueish-green.
The reason for this change is the formation of copper (II) chloride in the reaction. The general reaction between a metal oxide and an acid can be written as:
Metal oxide + Acid Salt + Water
Write the chemical equation for the reaction between copper oxide and HCl and balance it.
In above reaction metal oxide reacts with acid to give salt and water. This reaction is similar to the reaction of a base with an acid
Reaction of base with non-metal oxide
the reaction between carbon dioxide and calcium hydroxide (lime water) Calcium hydroxide, which is a base, reacts with carbon dioxide to produce a salt and water. This reaction is similar to the reaction between a base and an acid. Thus we can conclude that carbon dioxide which is a non-metal oxide is acidic in nature. In general all nonmetal oxides are acidic in nature.
In both the reactions salt and water are the products. Both metallic oxides and metallic hydrides give salt and water when they react with an acid. Thus we can conclude that metal oxides are basic in nature like the metal hydroxides
What do acids have in common?
acids generate hydrogen gas on reacting with metals, so hydrogen seems to be common element to all acids
Prepare solutions of glucose, alcohol, hydrochloric acid and sulphuric acid, etc.,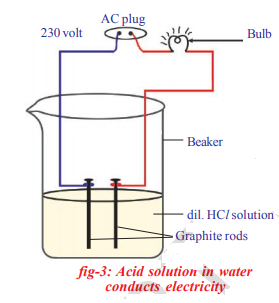
Connect two different coloured electrical wires to graphite rods separately in a 100 ml beaker
Connect free ends of the wire to 230 volts AC plug and complete the circuit by connecting a bulb to one of the wires. Now pour some dilute HCl in the beaker and switch on the current.
that the bulb glows only in acid solutions but not in glucose and alcohol solutions. Glowing of bulb indicates that there is flow of electric current through the solution. Acid solutions have ions and the moment of these ions in solution helps for flow of electric current through the solution
The positive ion (cation) present in HCl solution is H+. This suggests that acids produce hydrogen ions H+ in solution, which are responsible for their acidic properties. In glucose and alcohol solution the bulb did not glow indicating the absence of H+ ions in these solutions. The acidity of acids is attributed to the H+ ions produced by them in solutions.
Do acids produce ions only in aqueous solution? Let us test this
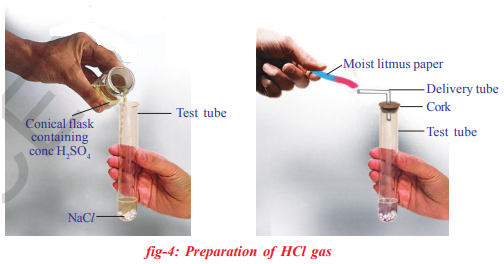
-Take about 1.0g of solid NaCl in a clean and dry test tube.
- Add some concentrated sulphuric acid to the test tube.
•What do you observe? Is there a gas coming out of the delivery tube? Let us write chemical equation for the above reaction
2NaCl+H_{2}SO_{4}\rightarrow 2HCl up arrow+Na_{2}SO_{4}
Test the gas evolved successively with dry and wet blue litmus paper.
In which case does the litmus paper change colour?
•What do you infer from the above observation? You can conclude that dry HCl gas (Hydrogen chloride) is not an acid because you have noticed that there is no change in colour of dry litmus paper but HCl aqueous solution is an acid because wet blue litmus paper turned into red.
Note : If the climate is very humid, pass the gas produced through a guard tube (drying tube) containing calcium chloride to dry the gas.
•Can you write the chemical equation for the reaction takes place at the mouth of delivery tube?
Laboratory precautions : Observe the following pictures. Did you find any problems with this? When ever you work with concentrated solutions it is very important to use test tube holder. It is very dangerous to work with bare hands in the laboratory
 The HCl gas evolved at delivery tube dissociates in presence of water to hydrogen ions. In the absence of water dissociation of HCl molecules do not occur.
The HCl gas evolved at delivery tube dissociates in presence of water to hydrogen ions. In the absence of water dissociation of HCl molecules do not occur.
The dissociation of HCl in water is shown below
HCl+H_{2}O\rightarrow H_{3}O+Cl-
Hydrogen ions cannot exist as bare ions. They associate with water molecules and exist as hydrated ions with each H+ attached by 4 to 6 water molecules. For this we represent H+ as hydronium ion, H3O+.
(H+)+ H_{2}O \rightarrow H_{3}O+
You have learnt that acids give H_{3}O+
Let us see what happens when a base is dissolved in water.
On dissolving bases in water produces hydroxide ( OH^{-}
What do you observe when water is mixed with acid or base?
-Take 10 ml water in a test tube.
-Add a few drops of concentrated H_{2}so_{4}
-Touch the bottom of the test tube. The process of dissolving an acid or a base in water is an exothermic process. Care must be taken while mixing concentrated nitric acid or sulphuric acid with water. The acid must always be added slowly to water with constant stirring. If water is added to a concentrated acid, the heat generated may cause the mixture to splash out and cause burns. The glass container may also break due to excessive local heating.
The process of dissolving an acid or a base in water is an exothermic process. Care must be taken while mixing concentrated nitric acid or sulphuric acid with water. The acid must always be added slowly to water with constant stirring. If water is added to a concentrated acid, the heat generated may cause the mixture to splash out and cause burns. The glass container may also break due to excessive local heating.
Mixing an acid or base with water result in decrease in the concentration of ions ( H_{3}O^{+}OH^{-}
Strength of acid or base
A test to know whether the Acid is strong or weak.
- Take two beakers A and B.
- Fill the beaker A with dil. CH_{3}COOH
with dil. HCl (Hydrochloric Acid)
through the solutions in separate beakers. What do you observe? Explain your observations. The universal indicator can also be used to know the strength of acid or base. Universal indicator is a mixture of several indicators. The universal indicator shows different colours at different concentrations of hydrogen ions in a solution.
pH scale
A scale for measuring hydrogen ion concentration in a solution is called pH scale. (The ‘p’ in pH stands for ‘Potenz’. In German Language ‘Potenz’ is power). pH value of a solution is simply a number which indicates the acidic or basic nature of a solution. The pH of neutral solutions is 7. Values less than 7 on the pH scale represent an acidic solution. As the pH value increases from 7 to 14, it represents a decrease in H3OH_{3}o^{+}
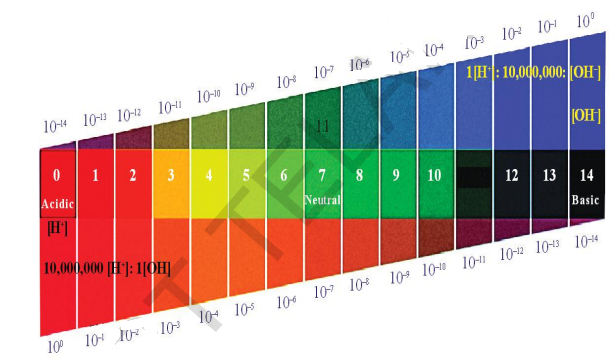
Importance of pH in everyday life
1.Are plants and animals pH sensitive ?
Living organisms can survive only in a narrow range of pH change. When pH of rain water is less than 5.6, it is called acid rain. When acid rain flows in to the rivers, it lowers the pH of the river water, the survival of aquatic life in such rivers becomes difficult.
2.Is pH change cause of tooth decay?
Tooth decay starts when the pH of the mouth is lower than 5.5. Tooth enamel, made of calcium phosphate is the hardest substance in the body. It does not dissolve in water, but is corroded when the pH in the mouth isbelow 5.5. Bacteria present in the mouth produce acids by degradation ofsugar and food particles remaining in the mouth. Using tooth pastes, which are generally basic neutralize the excess acid and prevent tooth decay.
3.pH in our digestive system:
It is very interesting to note that our stomach produces hydrochloric acid. It helps in the digestion of food without harming the stomach. During indigestion the stomach produces too much acid and this causes pain and irritation.
4.pH of the soil
Plants require a specific pH range for their healthy growth. To find out the pH required for the healthy growth of a plant, you can collect the soil samples from various places and check the pH in the manner described below in the following activity. Also you can note down what type of plants are growing in the region from which you have collected the soil.
Self defense by animals and plants through chemical war fare?
Have you ever been stung by a honey-bee? Bee sting leaves an acid which causes pain and irritation. Use of a mild base like baking soda on the stung area gives relief. Stinging hair of leaves of nettle plant, inject methanoic acid (formic acid) causing burning pain. A traditional remedy is rubbing the area with the leaf of the dock plant, which often grows besides the nettle in the wild.
More about salts
In the previous sections you have studied the formation of salts by the neutralization reaction of base with an acid. Let us understand more about the preparation, properties and uses of salts
Family of salts
- Write the formulae of the following salts.
- Potassium sulphate, sodium sulphate, calcium sulphate, magnesium
-sulphate, copper sulphate, sodium chloride, sodium nitrate, sodium
-corbonate and ammonium chloride.
- Identify the acids and bases from which the above salts are obtained.
- Salts having the same positive or negative radicals belong to a
family. For example, NaCl and Na_{_{2}}SO_{4}
How many families can you identify among the salts given above?
pH of Salts
- Collect the salt samples like, sodium chloride, aluminum chloride, copper sulphate, sodium acetate, ammonium chloride, sodium hydrogen carbonate and sodium carbonate.
- Dissolve them in distilled water.
- Check the action of these solutions with litmus papers.
- Find the pH using pH paper (universal indicator).
- Classify them into acidic, basic or neutral salts?
- Identify the acid and base used to form the above salts.
-Record your observations in Table - given below.
Salts of a strong acid and a strong base are neutral and the pH value is The salts of a strong acid and weak base are acidic and the pH value is less than 7. The salts of a strong base and weak acid are basic in nature and the pH value is more than 7.
Chemicals from common salt
Salts are the ionic compounds which are produced by the neutralization of acid with base. Salts are electrically neutral. There are number of salts but sodium chloride is the most common among them. Sodium chloride is also known as table salt or common salt. Sodium chloride is used to enhance the taste of food. These deposits oflarge crystals are often brown due to impurities. This is called rock salt. Beds of rock salt were formed when seas of bygone ages dried up. Rocksalt is mined like coal.
Common salt – A raw material for chemicals
The common salt is an important raw material for various materials of daily use, such as sodium hydroxide, baking soda, washing soda, bleaching powder and many more. Let us see how one substance is used for making all these different substances.
Sodium hydroxide from common salt
When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chloro-alkali process – because of the products formed chloro for chlorine and alkali for sodium hydroxide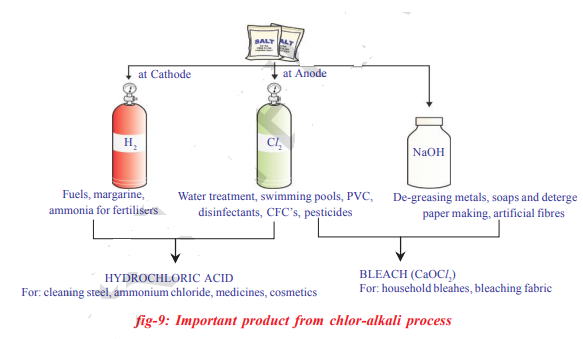 2MaCl+2H_{2}O\rightarrow 2NaOH+Cl_{2}+H_{2}
2MaCl+2H_{2}O\rightarrow 2NaOH+Cl_{2}+H_{2}
Chlorine gas is given off at the anode and hydrogen gas at the cathode, sodium hydroxide solution is formed near the cathode the three products produced in this process are all useful Fig. shows the different uses of these products.
Bleaching Powder
You know that chlorine is produced during the electrolysis of aqueoussodium chloride (brine). This chlorine gas is used for the manufacture of on dry slaked lime [ CaOH_{2}
Ca(OH_{2})+HCl_{2}\rightarrow CaOCl_{2}+H_{2}O
Uses of Bleaching Powder:
1.Used as an oxidizing agent in many chemical industries.
2.Used for disinfecting drinking water to make it free of germs.
3.Used as a reagent in the preparation of chloroform
Baking soda
Baking soda is sometimes added for faster cooking. The chemical name of the compound is sodium hydrogen carbonate . It is prepared as follows:
NaCl+H_{2}O+CO_{2}+NH_{3}\rightarrow NH_{4}Cl+NaHCO_{3}
Can you find the pH of sodium hydrogen carbonate as you have done Can you predict the reason for using NaHCO_{3}
Baking soda is a mild non-corrosive base. The following reaction takes place when it is heated during cooking.
2NaHCO_{3}\rightarrow Na_{2}Co_{3}+H_{2}O+CO_{2}
Uses of sodium hydrogen carbonate
1.Sodium hydrogen carbonate is also an ingredient in antacids.
2.It is also used as soda-acid in fire extinguishers
3.It acts as mild antiseptic.
Washing soda (sodium carbonate)
Another chemical that can be obtained from sodium chloride is
Na_{2}CO_{3}.10H_{2}O
carbonate can be obtained by heating baking soda. Recrystallisation of
sodium carbonate gives washing soda. It is also a basic salt.
Na_{2}CO_{3}+10H_{2}O \rightarrow Na_{2}CO_{3}.10H_{2}O
Sodium carbonate and sodium hydrogen carbonate are useful chemicals for many industrial processes.
Uses of Washing soda
i) Sodium carbonate (washing soda) is used in glass, soap and paper industries.
ii) It is used in the manufacture of sodium compounds such as borax.
Removing water of crystallization
Take a few crystals of copper sulphate in a dry test tube and heat the test tube.
- What change did younotice in the colour of the coppersulphate after heating?
Did you notice water dropletson sides of the test tube?Where did they come from?
Add 2-3 drops of water on thesample of copper sulphate obtainedafter heating.
What do you observe? Is the blue colour of copper sulphate restored?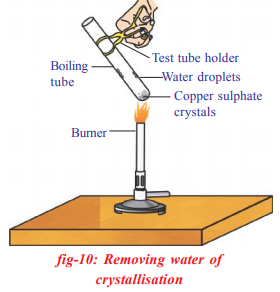 In the above activity copper sulphate crystals which seem to be dry contain the water of crystallization, when these crystals are heated, water present in crystals is evaporated and the salt turns white. When the crystals are moistened with water, the blue colour reappears. Water of crystallization is the fixed number of water molecules present in one formula unit of a salt. Five water molecules are present in one formula unit of copper sulphate. Chemical formula for hydrated copper sulphate is SUSO_{4}.5H_{2}O
In the above activity copper sulphate crystals which seem to be dry contain the water of crystallization, when these crystals are heated, water present in crystals is evaporated and the salt turns white. When the crystals are moistened with water, the blue colour reappears. Water of crystallization is the fixed number of water molecules present in one formula unit of a salt. Five water molecules are present in one formula unit of copper sulphate. Chemical formula for hydrated copper sulphate is SUSO_{4}.5H_{2}O
Now you would be able to answer the question whether the molecule
of Na_{2}CO_{3}.10H_{2}O
Another salt which possesses water of crystallisation is gypsum. It has two water molecules in its crystals and the formula is CaSO_{4}.2H_{2}O
Let us see the use of this salt.
Plaster of paris (CaSO_{4}.\frac{1}{2}H_{2}O)
On careful heating of gypsum (CaSO_{4}.\frac{1}{2}H_{2}O
CaSO_{4}.\frac{1}{2}H_{2}O+1\frac{1}{2}H_{2}O\rightarrow CaSO_{4}.2H_{2}O
note: You might have noticed that only half a water molecule is shown to be attached as water of crystallisation.
•How can you get half a water molecule? It is written in this form because two formula units of CaSO_{4}
•Try to collect the information for calling calcium sulphate hemihydrates as Plaster of Paris.



0 Doubts's