- Introduction
- Physical nature of matter
- Characteristics properties of matter
- States of matter
- Inter conversion of three states of the matter
- Can Matter Change its State?
- Sublimation of NH4CL
- Effect of change of Pressure
- Factors affecting evaporation
- How Does Evaporation Cause Cooling
- Question and Answers
- Matter in Our Surroundings
- Introduction
- What are the Mixtures
- What is a Mixture?Activity
- Concentration of a solution
- What are the solution and its properties?
- solubility
- What is a suspension and its properties
- calculating concentration
- What is a colloidal solution and its properties
- Based on physical states of collides
- Separation of the components of mixtures
- Separation of cream from milk
- Separating two immiscible liquids
- Compounds
- Pure Substances
- How can we obtain different gases from air?
- How can we obtain pure copper sulphate from an impure sample?
- Physical and chemical changes
- Question and Answers
- Matter around Us
- Introduction
- Laws of chemical combination
- Laws of constant proportion
- What is an Atom?
- symbols of atoms of different elements
- Atomic mass
- Dalton’s atomic theory
- What is a Molecule ?, Molecules of elements
- Molecules of compounds
- What is an Ion ?
- Writing chemical formula,simple compounds
- Molecular Mass and MoleConcept
- Mole concept
- Draw backs of dalton atomic model
- Problems on mole concept | part- 1
- Problems on mole concept | part-2
- Problems on mole concept | part-3
- Exercise Problems on atoms and molecules
- Question and Answers
- Atoms and Molecules
- Introduction
- Charged Particles in Matter
- The Structure of an Atom
- Discovery of Neutrons (By J. Chadwick)
- Atomic Models
- Thomson's Atomic Model
- Rutherford's Atomic Model
- Assumption made by Neil Bohr
- Distribution Of Electrons In Various Shells
- Valency
- Atomic Number and Mass Number
- Isotopes
- Question and Answers
- Structure Of The Atom
Biogeochemical Cycles
Biogeochemical Cycles
Biogeochemical cycles are repeated circulation of biogeochemical (water, nitrogen, oxygen etc) between abiotic and biotic components of the environment which result in their repeated withdrawal and replacement of their pool.
Water cycle:
1. Water is an essential and a principle component of living beings and also vita for life processes. It forms 60% to 90% of self-contained.
2. The whole process in which water evaporates and falls on the land as rain and water flows back into the sea by a river is known as the water cycle.
3. All of the water that falls on the land does not immediately flow back into the sea. Some of it seeps into the soil and become part of the underground reservoir of freshwater.
4. Some of these underground water finds its way to the surface through springs or we bring it to the surface for our use through wells and tube wells.
5. Rain may fall directly into the ocean, some water flows into the river and returns to the sea.
6. Water is also used by terrestrial animals and plants for various life processes.
7. Plants add water to the air as water vapors by transpiration. Animals return water to the air as vapors by respiration or to the soil as fluid by excretion.
8. So, there is a constant exchange of water between air, land, sea and between living organisms and their environment.
Oxygen cycle:
1. Oxygen is an essential component of all biomolecules. It is also required as such by all organisms that respire aerobically.
2. It is found in the elemental form in the atmosphere to the extent of 21%.it also occurs in combines forms as carbon dioxide, water, and it the earth’s crust as oxides of metals, carbonates, sulphate, etc.
3. Oxygen from the atmosphere is used up in 3 processes namely combustion, respiration and in the formation of oxides of nitrogen.
4. Its returns to the atmosphere by only one major process called photosynthesis.
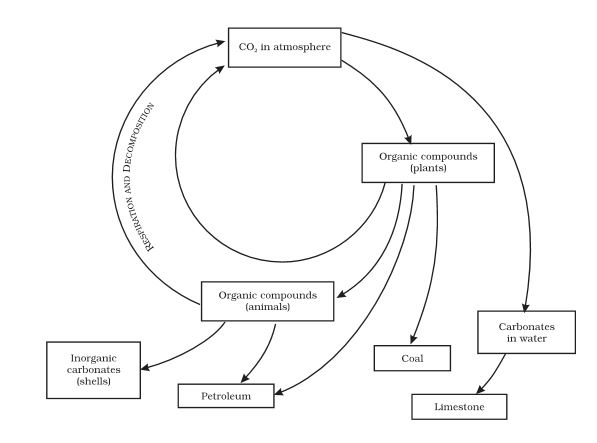
Carbon cycle:
1. The carbon is an important constituent of organic compounds found in all living beings in the form of carbohydrates, fats, proteins, and nucleic acid.
2. It occurs in the elemental form as diamond and graphite.
3. The endoskeletons of various animals are also formed from carbonates salts. It is found as carbon dioxide in the atmosphere.
4. Carbon is incorporated into lie forms through the basic process of photosynthesis. This process converts carbon dioxide from the atmosphere or dissolved in water into a glucose molecule.
5. The process of respiration converts glucose back into carbon dioxide. This carbon dioxide then goes back into the atmosphere.
6. Another process that adds to the carbon dioxide in the atmosphere is the process of combustion where fuels are burnt to provide energy for various needs. So, there is a constant cycling of carbon in the environment by various living and non-living beings.
Nitrogen cycle:
Importance of nitrogen:
Nitrogen gas makes up 78% of our atmosphere and nitrogen is also a part of many molecules essential to life like proteins, nucleic acids and some vitamins. Nitrogen is found in other biologically important compounds such as alkaloids and urea. So, nitrogen is an essential nutrient for all life forms.
Cycle:
1. Other than few forms of bacteria life forms are not able to convert the comparatively inert nitrogen molecule into forms like nitrates and nitrites which can be taken up and used to make the required molecules.
2. The conversion of inert nitrogen to nitrates and nitrites can be done either by industrial nitrogen fixation or by some nitrogen-fixing bacteria such as Azotobacter (occurs freely in soil) and Rhizobium (occurs in root nodules of leguminous plants as pea etc). The process of fixation of nitrogen is called nitrogen fixation.
3. In a physical process such as during lightning, the high temperature and pressure created in the air convert nitrogen into oxide of nitrogen. These oxides dissolve in water to give nitric and nitrous acids and fall on land along with rain (acid rain). These are then utilized by various life forms.
4. The plant generally takes up nitrates and nitrites and convert them into amino acids which are used to make proteins. These proteins and other complex compounds are subsequently consumed by animals. Once the animals or plants die, other bacteria in the soil convert the various compounds of nitrogen back into nitrates and nitrites. A different type of nitrogen releasing bacteria converts the nitrates and nitrites into elemental nitrogen.
5. So, there is a nitrogen cycle in nature which maintains the overall amount of nitrogen constant in the atmosphere, soil, and water.




0 Doubts's