- classification of Elements
- Dobereiner’s law of Triads
- Limitations
- Mendeleeff’s Periodic Table
- Salient features and achievements of the Mendeleeff’s periodic table
- Limitations of Mendeleeff’s periodic table
- Modern Periodic Table
- Groups,
- Periods
- Metals and Non metals
- Atomic radius
- Periodic properties of the elements in the modern table
- Ionization Energy
- Electronegativity
- Metallic and Non-Metallic Properties
- Short answer Questions
- Question And Answers
- Synopsis
- Quizz
- Introduction
- Lewis Electron Dot Symbols
- Ionic Compounds: Electrons Transferred
- Formation of Ionic Compounds
- The arrangement of ions in ionic compounds
- Covalent bond
- VSEPR theory
- Valence bond theory
-
Formation of Molecules









- Hybridisation
- Properties of ionic and covalent compounds
-
Formation of , water molecule







- Synopsis
- Question And Answers
- Quizz
Synopsis
Synopsis
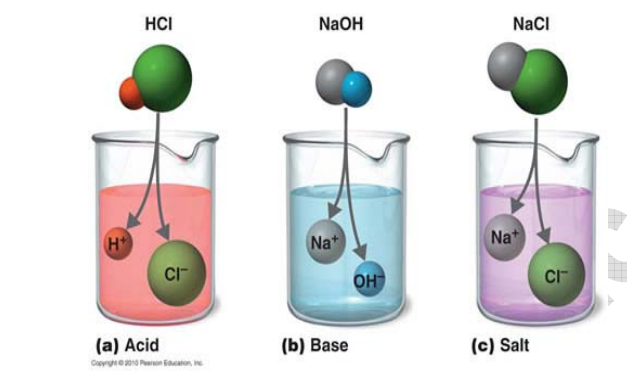
Substances are classified into acids, bases and salts depending on their chemical behavior and properties. Acids are of sour taste and turn blue litmus to red. Bases are soapy to touch and turn red litmus to blue. According to Lewis, electron pair acceptor is an acid and electron pair donor is a base.
The strength of acid or base depends on the concentration of















A scale for measuring hydrogen ion concentration in a solution is called 'pH' scale. The pH scale ranges from 0 to 14. Acid pH value varies from 0 to 7. Neutral substances have PH value 7.pH value is 7 to 14 for Base.
'When acid reacts with base, it forms salt and water. Sodium chloride is the most common salt. Sodium hydroxide, Bleaching powder, baking soda, washing soda are some of the salts obtained from common salt. Plaster of Paris is obtained from gypsum.
Please Login For Further Details...
Ask Doubts's Cancel reply
© - 2018 KlassPM Educational Consultancy Pvt Ltd. All Rights Reserved.



0 Doubts's