- classification of Elements
- Dobereiner’s law of Triads
- Limitations
- Mendeleeff’s Periodic Table
- Salient features and achievements of the Mendeleeff’s periodic table
- Limitations of Mendeleeff’s periodic table
- Modern Periodic Table
- Groups,
- Periods
- Metals and Non metals
- Atomic radius
- Periodic properties of the elements in the modern table
- Ionization Energy
- Electronegativity
- Metallic and Non-Metallic Properties
- Short answer Questions
- Question And Answers
- Synopsis
- Quizz
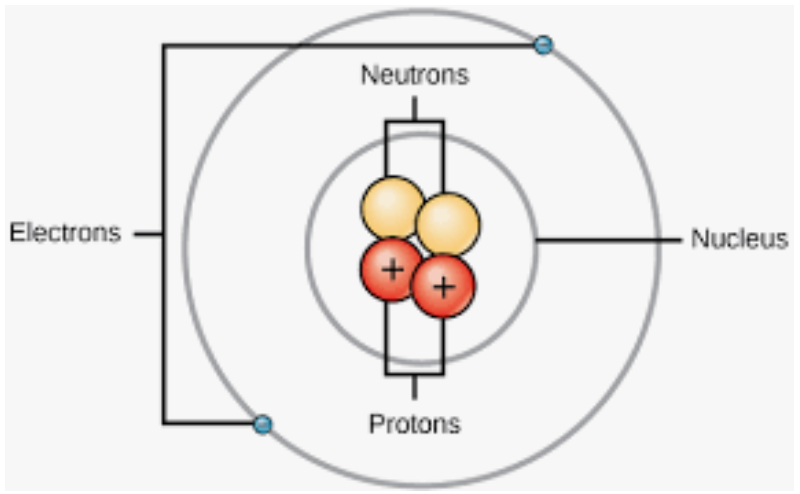
- Introduction
- Lewis Electron Dot Symbols
- Ionic Compounds: Electrons Transferred
- Formation of Ionic Compounds
- The arrangement of ions in ionic compounds
- Covalent bond
- VSEPR theory
- Valence bond theory
-
Formation of O_2, N_2, CH_4Molecules
- Hybridisation
- Properties of ionic and covalent compounds
-
Formation of BF_3,NH_3, water molecule
- Synopsis
- Question And Answers
- Quizz
Synopsis
Synopsis
Energy propagates as electromagnetic waves and can have a wide variety of wavelengths. The entire range of wavelengths is known as the electromagnetic spectrum.
Max Planck broke with the continuous energy tradition of electromagnetic energy by assuming that the energy is always emitted in multiples of "hv" with his Planck's constant
which has the value 6.626\times 10^{-34}J-S
.Where "v" is the frequency.
Using Planck's theory and Rutherford model, Bohr proposed his famous atomic model. The defects of this model were rectified by Somerfield. This Bohr- Somerfield model, though successful in accounting for the fine line structure of hydrogen atomic spectra, failed to provide a satisfactory picture of the structure of the atom in general.
For finding the electron in the space around the nucleus, quantum numbers are proposed by different scientists.
There are four quantum numbers.Those are
- Principal quantum number (n).
- The angular-momentum quantum number (1).
- The magnetic quantum number (ml).
- Spin Quantum number (ms).
The Pauli Exclusion Principle states that two electrons of the same atom can have all four quantum numbers the same.
Aufbau Principle gives information about how orbitals are filled in the order of increasing energy.
Please Login For Further Details...
Ask Doubts's Cancel reply
© - 2018 KlassPM Educational Consultancy Pvt Ltd. All Rights Reserved.



0 Doubts's