- classification of Elements
- Dobereiner’s law of Triads
- Limitations
- Mendeleeff’s Periodic Table
- Salient features and achievements of the Mendeleeff’s periodic table
- Limitations of Mendeleeff’s periodic table
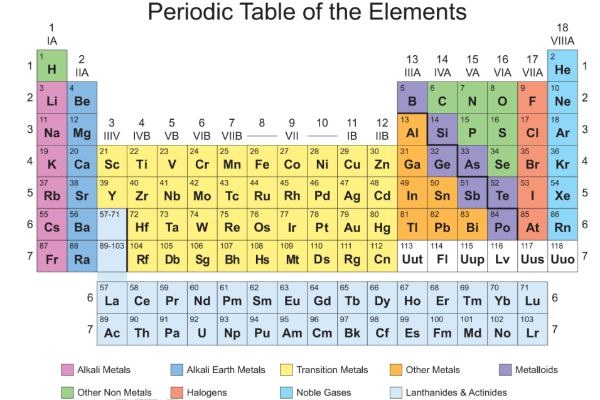
- Modern Periodic Table
- Groups,
- Periods
- Metals and Non metals
- Atomic radius
- Periodic properties of the elements in the modern table
- Ionization Energy
- Electronegativity
- Metallic and Non-Metallic Properties
- Short answer Questions
- Question And Answers
- Synopsis
- Quizz
- Introduction
- Lewis Electron Dot Symbols
- Ionic Compounds: Electrons Transferred
- Formation of Ionic Compounds
- The arrangement of ions in ionic compounds
- Covalent bond
- VSEPR theory
- Valence bond theory
- Formation of $$O_2, N_2, CH_4$$ Molecules
- Hybridisation
- Properties of ionic and covalent compounds
- Formation of $$BF_3,NH_3$$, water molecule
- Synopsis
- Question And Answers
- Quizz
Synopsis
Synopsis
From the earliest times, scientists have been trying to classify the available elements on the basis of their properties.
Dobereiner proposed the law of triads'. Newland proposed the law of octaves. Mendeleeff divides the elements into groups on the basis of "atomic weights". The limitations in Mendeleev's periodic table are removed in the modern periodic table.
In modern periodic table eighteen groups and seven periods. There are 's' block, `p' block, `d' block and 'f' block elements.
Atomic radius, Ionization energy, electron affinity, Electro negativity are the main characteristic of the enlacements both in groups and periods
Please Login For Further Details...
Ask Doubts's Cancel reply
© - 2018 KlassPM Educational Consultancy Pvt Ltd. All Rights Reserved.



0 Doubts's